Coffee is one of the most popular beverages in the world. It is consumed by millions of people every day, and for many, it is an essential part of their morning routine. However, some people prefer to avoid caffeine due to its negative effects on their health. That's where decaffeinated coffee comes in. Decaf coffee is made by removing caffeine from coffee beans. There are several methods used to decaffeinate coffee, but they all involve the use of chemicals. In this article, we will discuss the chemicals used to decaffeinate coffee.
What is Decaffeination?
Decaffeination is the process of removing caffeine from coffee beans. The process is done to produce decaf coffee, which has a lower caffeine content compared to regular coffee. Decaffeination can be done using various methods, including the use of chemicals, water, or carbon dioxide.

Chemicals Used To Decaffeinate Coffee
The most common chemicals used to decaffeinate coffee are methylene chloride and ethyl acetate. Methylene chloride is a colorless liquid with a sweet smell. It is a popular solvent used in many industries, including the coffee industry. Ethyl acetate is another solvent used in the coffee industry. It is a colorless liquid with a fruity smell. It is used in the food and beverage industry as a flavoring agent.

The Decaffeination Process Using Methylene Chloride
The decaffeination process using methylene chloride starts by soaking the coffee beans in hot water. This process removes the caffeine and other soluble compounds from the beans. The water is then separated from the beans, and the beans are soaked in methylene chloride, which dissolves the caffeine. The caffeine is then removed from the methylene chloride using steam, leaving behind decaf coffee beans.

The Decaffeination Process Using Ethyl Acetate
The decaffeination process using ethyl acetate is similar to the process using methylene chloride. The coffee beans are first soaked in hot water to remove the caffeine and other soluble compounds. The water is then separated from the beans, and the beans are soaked in ethyl acetate. The ethyl acetate dissolves the caffeine, which is then removed using steam, leaving behind decaf coffee beans.
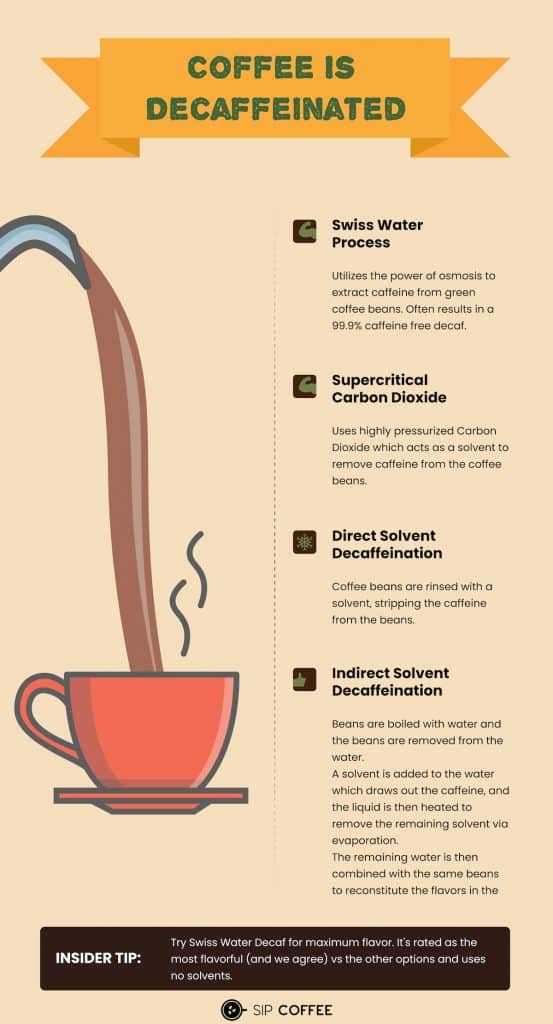
Other Methods of Decaffeination
Aside from using methylene chloride and ethyl acetate, there are other methods of decaffeination. These include the Swiss water process, which uses only water to remove caffeine from coffee beans, and the carbon dioxide process, which uses carbon dioxide to dissolve caffeine.
Is Decaffeinated Coffee Safe?
Decaffeinated coffee is generally considered safe for consumption. The chemicals used in the decaffeination process are removed from the beans before they are roasted, so there is no risk of consuming them. However, some people may be sensitive to residual chemicals, so it's best to choose decaf coffee that uses the Swiss water process or the carbon dioxide process.
Conclusion
Decaffeinated coffee is a great option for people who want to enjoy the taste of coffee without the negative effects of caffeine. The decaffeination process involves the use of chemicals such as methylene chloride and ethyl acetate, but these chemicals are removed from the beans before they are roasted. If you're sensitive to residual chemicals, it's best to choose decaf coffee that uses the Swiss water process or the carbon dioxide process.